|

|
Asacol® (mesalamine) Delayed-Release Tablets,
available only by prescription from your doctor, is the only sulfa-free
medication indicated to both:
- treat mild to moderate flare-ups of
ulcerative colitis
- maintain remission of ulcerative colitis
|

Asacol is proven to treat mild to moderate flare-ups of ulcerative colitis.
In a six-week clinical study of flare-ups, Asacol was evaluated for its
ability to reduce ulcerative colitis symptoms, including rectal bleeding,
number of bowel movements, and abdominal pain. After three weeks of
therapy, rectal bleeding and number of bowel movements were reduced.

Asacol is also proven to maintain remission. In a six-month study, Asacol
extended patients' time in remission when taken continuously at the
maintenance dose, as compared to placebo (sugar pill).
Talk with your
doctor to see if remission therapy is right for you. Responses to
remission therapy can vary. In addition, drug therapy may cause side
effects.
Ulcerative colitis rarely goes into permanent remission. The risk
of flare-ups can be substantially reduced by continued use of Asacol at
the maintenance dose as directed by your doctor.

You should not take Asacol if you have been told by a health care
professional that you have an increased sensitivity, or hypersensitivity,
to salicylates, such as aspirin, or to any of the components of the
Asacol tablet. Talk to your doctor if you have any questions about
increased sensitivity to salicylates or if your condition worsens while
taking Asacol.
|


|
There are some important precautions you should be aware of
regarding Asacol therapy, including:
General. If you have pyloric stenosis (a narrowing of
the outlet from the stomach that causes contents of the stomach to remain
there for a longer period of time), you should tell your doctor before
using Asacol. Pyloric stenosis may keep the Asacol tablet from reaching
the colon as quickly as it normally would.
Pregnant or nursing mothers. If you are
pregnant, become pregnant, or are a nursing mother, talk with your doctor
before using Asacol therapy.
Recommended periodic monitoring.
Your doctor may require certain tests to check your kidney function
before starting Asacol therapy and periodically while you continue Asacol
therapy. These tests will make sure the medication is not harming your
kidneys.
Kidney function. If you have a history of
kidney impairment or kidney disease, you should tell your doctor before
using Asacol, as it may worsen your kidney condition.
Worsening of the symptoms. Worsening of the symptoms
of ulcerative colitis was reported in 3% of Asacol-treated people in
clinical trials.
Reaction to sulfasalazine or mesalamine.
Some people who have had a reaction to sulfasalazine may have a similar
reaction to Asacol or other products containing mesalamine. Such a
reaction may include worsening of ulcerative colitis symptoms (i.e.,
cramping, abdominal pain, and bloody diarrhea), and occasionally fever,
headache, general discomfort, itching, rash, and conjunctivitis (an
inflammation of part of the eye).
Talk with your doctor about your medical history and if you have
any questions about your medication. It's also important to visit your doctor
periodically to monitor your condition and discuss how your treatment
plan is working for you.
|
For more
information, please refer to the Precautions
section of the 


|

In clinical studies comparing people taking Asacol to those
taking a sugar pill (placebo), there was no significant difference in the
number of people reporting side effects. Please note, however, Asacol is
a medication and side effects may occur with its use. In studies of
flare-ups, some people taking Asacol reported headache, abdominal pain,
general pain, rash, upset stomach, and worsening of ulcerative colitis
symptoms. In a maintenance study, headache, runny nose, general pain,
sore throat, infection, and nervousness were reported.
Some serious side effects may occur. Periodic visits to your
doctor are important. Contact your doctor if you have any questions or if
you are experiencing any side effects while taking Asacol.
|


|

Asacol has a favorable safety profile based on a six-month
maintenance study and in clinical studies of flare-ups. Results included:
- No
overall significant changes in kidney function, liver function, or
blood cell tests. Since the potential for kidney problems exists,
periodic monitoring by your doctor is recommended.
- The
number of people withdrawing from studies as a result of side
effects was low (less than 4%) and similar among people taking sugar
pill (placebo). No one withdrew from this study as a result of an
abnormal lab test.
|


|
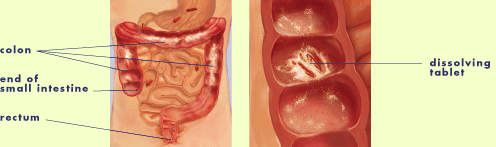
Asacol tablets are designed with a special outer coating that
targets the delivery of the medication to the colon where it is needed.
- This unique delivery system allows the tablet to travel
in one piece through the digestive tract until it reaches the
end of the small intestine and beginning of the colon.
- At this location, the tablet's special coating begins to
dissolve and release the active ingredient, 5-ASA, for activity
throughout the entire colon.
- The active ingredient acts right at the site to
reduce inflammation of the inner lining of the colon and rectum.
|
When taking Asacol, be sure to swallow your tablets whole - do
not break or chew them. Chewing breaks the special outer coating.
In clinical studies, a small percentage of people (2 to 3%) have
reported passage in the stool of what appears to be whole tablets,
fragments of tablets, or tablet shells. If this occurs repeatedly,
contact your doctor.
|
|