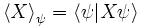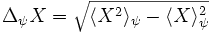Uncertainty principle
From Wikipedia, the free encyclopedia
In quantum physics, the Heisenberg uncertainty principle or just Uncertainty principle (sometimes also the Heisenberg indeterminacy principle - a name given to it by Niels Bohr) states that one cannot measure values (with arbitrary precision) of certain conjugate quantities, which are pairs of observables of a single elementary particle. These pairs include the position and momentum. Mathematics provides a positive lower bound for the product of the uncertainties of measurements of the conjugate quantities. The uncertainty principle is one of the cornerstones of quantum mechanics and was discovered by Werner Heisenberg in 1927.
The Uncertainty principle follows from the mathematical definition of operators in quantum mechanics; it is a theorem of functional analysis. It is often confused with the observer effect.
Contents |
Overview

The concept of probability distributions pervades the science of measurement. Until the beginning of the discovery of quantum physics, it was thought that the only uncertainty in measurement was caused by the limitations of a measuring tool's precision. But it is now understood that no treatment of any scientific subject, experiment, or measurement is said to be accurate without disclosing the nature of the probability distribution (sometimes called the error) of the measurement. Uncertainty is the characterization of the relative narrowness or broadness of the distribution function applied to a physical observation.
Illustrative of this is an experiment in which a particle is prepared in a definite state and two successive measurements are performed on the particle. The first one measures the particle's position and the second immediately after measures its momentum. Each time the experiment is performed, some value x is obtained for position and some value p is obtained for momentum. Depending upon the precision of the instrument taking the measurements, each successive measurement of the positions and momenta respectively should be nearly identical, but in practice they will exhibit some deviation due to constraints of measurement using a real world instrument that is not infinitely precise. However, Heisenberg showed that, even in theory with a hypothetical infinitely precise instrument, no measurement could be made to arbitrary accuracy of both the position and the momentum of a physical object.
The Heisenberg uncertainty principle (developed in an essay published in 1927) provides a quantitative relationship between the uncertainties of the hypothetical infinitely precise measurements of p and x as measured by the sizes of their distributions in the following way: If the particle state is such that the first measurement yields a dispersion of values Δx, then the second measurement will have a distribution of values whose dispersion Δp is at least inversely proportional to Δx. For the limiting case, the constant of proportionality is derivable using commutator arithmetic. It is equal to Planck's constant divided by 4π.
This stipulates that the product of the uncertainties in position and momentum is equal to or greater than about 10 − 35 joule-seconds. Therefore, the product of the uncertainties only becomes significant for regimes where the uncertainty in position or momentum measurements is small. Thus, the uncertainty principle governs the observable nature of atoms and subatomic particles while its effect on measurements in the macroscopic world is negligible and can be usually ignored.
The Heisenberg uncertainty relations are a theoretical bound over all measurements. They hold for so-called ideal measurements, sometimes called von Neumann measurements. They hold even more so for non-ideal or Landau measurements.
Wave-particle duality and the relationship to the uncertainty principle
A fundamental consequence of the Heisenberg Uncertainty Principle is that no physical phenomena can be to arbitrary accuracy described as a "classic point particle" or as a wave but rather the microphysical situation is best described in terms of wave-particle duality. The uncertainty principle, as initially considered by Heisenberg, is concerned with cases in which neither the wave nor the point particle descriptions are fully and exclusively appropriate, such as a particle in a box with a particular energy value. Such systems are characterized neither by one unique "position" (one particular value of distance from a potential wall) nor by one unique value of momentum (including its direction). Any observation that determines either a position or a momentum of such a waveparticle to arbitrary accuracy - known as wavefunction collapse - is subject to the condition that the width of the wavefunction collapse in position, multiplied by the width of the wavefunction collapse in momentum, is constrained by the principle to be greater than or equal to Planck's constant divided by 4π.
Every measured particle in quantum mechanics exhibits wavelike behaviour so there is an exact, quantitative analogy between the Heisenberg uncertainty relations and properties of waves or signals. For example, in a time-varying signal such as a sound wave, it is meaningless to ask about the frequency spectrum at a single moment in time because the measure of frequency is the measure of a repetition recurring over a period of time. In order to determine the frequencies accurately, the signal needs to be sampled for a finite (non zero) time. This necessarily implies that time precision is lost in favor of a more accurate measurement of the frequency spectrum of a signal. This is analogous to the relationship between momentum and position, and there is an equivalent formulation of the uncertainty principle which states that the uncertainty of energy of a wave (directly proportional to the frequency) is inversely proportional to the uncertainty in time with a constant of proportionality identical to that for position and momentum.
Common incorrect explanation of the uncertainty principle
The uncertainty principle in quantum mechanics is sometimes erroneously explained by claiming that the measurement of position necessarily disturbs a particle's momentum. Heisenberg himself may have initially offered explanations which suggested this view. That this disturbance does not describe the essence of the uncertainty principle in current theory has been demonstrated above. The fundamentally non-classical characteristics of the uncertainty measurements in quantum mechanics were clarified due to the EPR paradox which arose from Einstein attempting to show flaws in quantum measurements that used the uncertainty principle. Instead of Einstein succeeding in showing uncertainty was flawed, Einstein guided researchers to examine more closely what uncertainty measurements meant and led to a more refined understanding of uncertainty. Prior to the publication of the EPR paper in 1935, a measurement was often visualized as a physical disturbance inflicted directly on the measured system, being sometimes illustrated as a thought experiment called Heisenberg's microscope. For instance, when measuring the position of an electron, one imagines shining a light on it, thus disturbing the electron and producing the quantum mechanical uncertainties in its position. Such explanations, which are still encountered in popular expositions of quantum mechanics, are debunked by the EPR paradox, which shows that a "measurement" can be performed on a particle without disturbing it directly, by performing a measurement on a distant entangled particle. Heisenberg's original argument used the 'old' quantum theory ( namely, the Einstein-deBroglie relations ) and provided a heuristic argument that the position and momentum observables were not simultaneously observable with infinite precision. The more modern uncertainty relations deal with independent measurements being done on an ensemble of systems.
Formulation and characteristics
Measurements of position and momentum taken in several identical copies of a system in a given state will vary according to known probability distributions. This is the fundamental postulate of quantum mechanics.
If we compute the uncertainty Δx of the position measurements and the standard deviation Δp of the momentum measurements, then
where
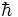 is the reduced Planck's
constant (Planck's constant divided by 2π).
is the reduced Planck's
constant (Planck's constant divided by 2π).
Heisenberg did not just use any arbitrary number to describe the minimum
standard deviation between position and momentum of a particle. Heisenberg knew
that particles behaved like waves and he knew that the energy of any wave is the
frequency multiplied by Planck's constant. In a wave, a cycle is defined by the
return from a certain position to the same position such as from the top of one
crest to the next crest. This actually is equivalent to a circle of 360 degrees,
or 2π radians. Therefore, dividing h by 2π describes a constant that when multiplied by the
frequency of a wave gives the energy of one radian. Heisenberg took 1/2 of
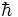 as his standard deviation. This can be written as
as his standard deviation. This can be written as 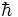 over 2 as above or it can be written using simple algebra and multiplying 1/2 x
h/(2π) as h/(4π). Normally
one will see
over 2 as above or it can be written using simple algebra and multiplying 1/2 x
h/(2π) as h/(4π). Normally
one will see 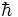 over 2 as this is simpler.
over 2 as this is simpler.
Two years earlier in 1925 when Heisenberg had developed his matrix mechanics the
difference in position and momentum were already showing up in the formula. In
developing matrix mechanics Heisenberg was measuring amplitudes of position and
momentum of particles such as the electron that have a period of 2π, like a cycle in a wave, which are called Fourier series variables.
When amplitudes of position and momentum are measured and multiplied together,
they give intensity. However, Heisenberg found that when the position and
momentum were multiplied together in that respective order or in the reverse
order, there was a difference between the two calculated intensities of
h/(2π). In other words, the two quantities position
and momentum did not commute. In 1927, to develop the standard deviation for the
uncertainty principle, Heisenberg took the gaussian
distribution or bell curve for the imprecision in the measurement of the
position q of a moving electron to the corresponding bell curve of the measured
momentum p. That gave the minimum standard deviation to be 1/2 of h/(2π), or, 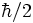 .
.
In some treatments, the "uncertainty" of a variable is taken to be the smallest width of a range which contains 50% of the values, which, in the case of normally distributed variables, leads to a larger lower bound of h/(2π) for the product of the uncertainties. Note that this inequality allows for several possibilities: the state could be such that x can be measured with high precision, but then p will only approximately be known, or conversely p could be sharply defined while x cannot be precisely determined. In yet other states, both x and p can be measured with "reasonable" (but not arbitrarily high) precision.
Expression of finite available amount of Fisher information
The uncertainty principle alternatively derives as an expression of the Cramér-Rao inequality of classical measurement theory. This is in the case where a particle position is measured. See Stam (1959). The mean-squared particle momentum enters as the Fisher information in the inequality. See also extreme physical information.
Common observables which obey the uncertainty principle
An uncertainty relation arises between any two observable quantities that can be defined by non-commuting operators. This means that the uncertainty principle arises in measuring the position and the velocity of an object, or in measuring the position and momentum of an object.
- The most common one is the uncertainty relation between position and momentum of a particle in space:
- The uncertainty relation between two orthogonal components of the total angular momentum operator of a particle is as follows:
-
- where i, j, k are distinct and Ji denotes angular momentum along the xi axis.
- The following uncertainty relation between energy and time is often presented in physics textbooks, although its interpretation requires more care because there is no operator representing time:
- The correct formulation and proof of this relation were suggested by L. I. Mandelshtam and I. E. Tamm in 1945. In 2005 D. A. Arbatsky generalized their result for relativistic systems.
The theorem
The uncertainty principle becomes a theorem in the theory of operators.
Theorem. For arbitrary symmetric operators A: H → H and B: H → H, and any element x of H such that A B x and B A x are both defined (so that in particular, A x and B x are also defined), then
This is an immediate consequence of the Cauchy-Bunyakovski-Schwarz inequality.
Consequently, the following general form of the uncertainty principle, first pointed out in 1930 by Howard Percy Robertson and (independently) by Erwin Schrödinger, holds:
This inequality is called the Robertson-Schrödinger relation.
The operator A B - B A is called the commutator of A, B and is denoted [A, B]. It is defined on those x for which A B x and B A x are both defined.
From the Robertson-Schrödinger relation, the following Heisenberg uncertainty relation is immediate:
Suppose A and B are two observables which are identified to self-adjoint (and in particular symmetric) operators. If B A ψ and A B ψ are defined then
where
is the operator mean of observable X in the system state ψ and
is the operator standard deviation of observable X in the system state ψ
The above definitions of mean and standard deviation are defined formally in purely operator-theoretic terms. The statement becomes more meaningful however, once we note that these actually are the mean and standard deviation for the measured distribution of values. See quantum statistical mechanics.
It may be evaluated not only for pairs of conjugate operators (e.g. those defining measurements of distance and of momentum, or of duration and of energy) but generally for any pair of Hermitian operators. There is also an uncertainty relation between the field strength and the number of particles which is responsible for the phenomenon of virtual particles.
Note that it is possible to have two non-commuting self-adjoint operators A and B which share an eigenvector ψ, in this case ψ represents a pure state in which it is predictable with probability one what the result of measuring A or B will be in spite of their not being simultaneously measurable.
History and interpretations
Main article: Interpretation of quantum mechanics
The Uncertainty Principle was developed as an answer to the question: How does one measure the location of an electron around a nucleus?
In the summer of 1922 Heisenberg met Niels Bohr, the founding father of quantum mechanics, and in September 1924 Heisenberg went to Copenhagen, where Bohr had invited him as a research associate and later as his assistant. In 1925 Werner Heisenberg laid down the basic principles of a complete quantum mechanics. In his new matrix theory he replaced classical commuting variables with non-commuting ones. Heisenberg's paper marked a radical departure from previous attempts to solve atomic problems by making use of observable quantities only. He wrote in a 1925 letter, "My entire meagre efforts go toward killing off and suitably replacing the concept of the orbital paths that one cannot observe." Rather than struggle with the complexities of three-dimensional orbits, Heisenberg dealt with the mechanics of a one-dimensional vibrating system, an anharmonic oscillator. The result was formulae in which quantum numbers were related to observable radiation frequencies and intensities. In March 1926, working in Bohr's institute, Heisenberg formulated the principle of uncertainty thereby laying the foundation of what became known as the Copenhagen interpretation of quantum mechanics.
Albert Einstein was not happy with the uncertainty principle, and he challenged Niels Bohr and Werner Heisenberg with a famous thought experiment (See the Bohr-Einstein debates for more details): we fill a box with a radioactive material which randomly emits radiation. The box has a shutter, which is opened and immediately thereafter shut by a clock at a precise time, thereby allowing some radiation to escape. So the time is already known with precision. We still want to measure the conjugate variable energy precisely. Einstein proposed doing this by weighing the box before and after. The equivalence between mass and energy from special relativity will allow you to determine precisely how much energy was left in the box. Bohr countered as follows: should energy leave, then the now lighter box will rise slightly on the scale. That changes the position of the clock. Thus the clock deviates from our stationary reference frame, and again by special relativity, its measurement of time will be different from ours, leading to some unavoidable margin of error. In fact, a detailed analysis shows that the imprecision is correctly given by Heisenberg's relation.
The term Copenhagen interpretation of quantum mechanics was often used interchangeably with and as a synonym for Heisenberg's Uncertainty Principle by detractors who believed in fate and determinism and saw the common features of the Bohr-Heisenberg theories as a threat. Within the widely but not universally accepted Copenhagen interpretation of quantum mechanics (i.e. it was not accepted by Einstein or other physicists such as Alfred Lande), the uncertainty principle is taken to mean that on an elementary level, the physical universe does not exist in a deterministic form—but rather as a collection of probabilities, or potentials. For example, the pattern (probability distribution) produced by millions of photons passing through a diffraction slit can be calculated using quantum mechanics, but the exact path of each photon cannot be predicted by any known method. The Copenhagen interpretation holds that it cannot be predicted by any method, not even with theoretically infinitely precise measurements.
It is this interpretation that Einstein was questioning when he said "I cannot believe that God would choose to play dice with the universe." Bohr, who was one of the authors of the Copenhagen interpretation responded, "Einstein, don't tell God what to do." Niels Bohr himself acknowledged that quantum mechanics and the uncertainty principle were counter-intuitive when he stated, "Anyone who is not shocked by quantum theory has not understood a single word."
The basic debate between Einstein and Bohr (including Heisenberg's Uncertainty Principle) was that Einstein was in essence saying: "Of course, we can know where something is; we can know the position of a moving particle if we know every possible detail, and thereby by extension, we can predict where it will go." Bohr and Heisenberg were saying the opposite: "There is no way to know where a moving particle is ever even given every possible detail, and thereby by extension, we can never predict where it will go."
Einstein was convinced that this interpretation was in error. His reasoning was that all previously known probability distributions arose from deterministic events. The distribution of a flipped coin or a rolled dice can be described with a probability distribution (50% heads, 50% tails). But this does not mean that their physical motions are unpredictable. Ordinary mechanics can be used to calculate exactly how each coin will land, if the forces acting on it are known. And the heads/tails distribution will still line up with the probability distribution (given random initial forces).
Einstein assumed that there are similar hidden variables in quantum mechanics which underlie the observed probabilities and that these variables, if known, would show that there was what Einstein termed "local realism", a description opposite to the uncertainty principle, being that all objects must already have their properties before they are observed or measured. For the greater part of the twentieth century, there were many such hidden variable theories proposed, but in 1964 John Bell theorized the Bell inequality to counter them, which postulated that although the behavior of an individual particle is random, it is also correlated with the behavior of other particles. Therefore, if the uncertainty principle is the result of some deterministic process in which a particle has local realism, it must be the case that particles at great distances instantly transmit information to each other to ensure that the correlations in behavior between particles occur. The interpretation of Bell's theorem explicitly prevents any local hidden variable theory from holding true because it shows the necessity of a system to describe correlations between objects. The implication is, if a hidden local variable is the cause of particle 1 being at a position, then a second hidden local variable would be responsible for particle 2 being in its own position - and there is no system to correlate the behavior between them. Experiments have demonstrated that there is correlation. In the years following, Bell's theorem was tested and has held up experimentally time and time again, and these experiments are in a sense the clearest experimental confirmation of quantum mechanics. It is worth noting that Bell's theorem only applies to local hidden variable theories; non-local hidden variable theories can still exist (which some, including Bell, think is what can bridge the conceptual gap between quantum mechanics and the observable world).
Whether Einstein's view or Heisenberg's view is true or false is not a directly empirical matter. One criterion by which we may judge the success of a scientific theory is the explanatory power it gives us, and to date it seems that Heisenberg's view has been the better at explaining physical subatomic phenomena.
The uncertainty principle in popular culture
The uncertainty principle is stated in popular culture in many ways, for example by stating that it is impossible to know both where an electron is and where it is going at the same time. This is roughly correct, although it fails to mention an important part of the Heisenberg principle, which is the quantitative bounds on the uncertainties.
The uncertainty principle is frequently, but incorrectly, confused with the "observer effect", wherein the observation of an event changes the event. The observer effect is an important effect in many fields, from electronics to psychology and social science.
In some science fiction stories, a device to circumvent the uncertainty principle is called a Heisenberg compensator, most famously in Star Trek for use on the transporter; however, it is not clear what compensating means.
The character, Phillip Richbourg, of Night Court got into a heated debate with Judge Harry about this very principle. He argued the "ball theory", claiming that if you roll a ball down a hill, and you know exactly when it has been exactly 10sec, and you can measure distance exactly, I don't see how you can't know where the ball is. It was very comical and fed in with the show's theme.
In Stephen Donaldson's Gap
Cycle science fiction book series, one of the characters postulates a
socio-political version of the uncertainty principle: namely, that by
determining his precise "location" in the current political landscape, he is
prevented from simultaneously calculating the likely direction of political
events in the near future.
In software programming, a Heisenbug is a software error that disappears or alters its characteristics when it is researched.
Humor
The unusual nature of Heisenberg's uncertainty principle, and its distinctive name, has made it the source of several jokes.
It is said that a popular item of graffiti at the physics department of university campuses is the slogan "Heisenberg may have been here."
In another uncertainty principle joke, a quantum physicist is stopped on the highway by a police officer who asks "Do you know how fast you were going, sir?", to which the physicist responds, "No, but I know exactly where I am!".
"How many Physicists does it take to change a light bulb?" "Only one, and all the Physicist has to do is observe the light bulb and he changes it."
The biggest flop since the Edsel... The Heisenbergmobile. The problem was that when you look at the speedometer you got lost.
This may be apocryphal; in the UK a physics grad has escaped a speeding fine, claiming that the HUP prohibited the court from proving the speed at which he was travelling and that he was there at the same time!
See also
- Quantum indeterminacy
- Basics of quantum mechanics
- Qubit Field Theory
- Ehrenfest theorem
- Correspondence principle
References
Textbooks
- Griffiths, David J. (2004). Introduction to Quantum Mechanics (2nd ed.). Prentice Hall. ISBN 013805326X.
- Omnes, Roland (1999). Understanding Quantum Mechanics. Princeton University Press. ISBN 0691004358.
- J. von Neumann, Mathematical Foundations of Quantum Mechanics, Princeton University Press, 1955.
- H. Weyl, The Theory of Groups and Quantum Mechanics, Dover Publications 1950.
Journal articles
- W. Heisenberg, "Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik", Zeitschrift für Physik, 43 1927, pp. 172-198. English translation: J. A. Wheeler and H. Zurek, Quantum Theory and Measurement Princeton Univ. Press, 1983, pp. 62-84.
- L. I. Mandelshtam, I. E. Tamm "The uncertainty relation between energy and time in nonrelativistic quantum mechanics", Izv. Akad. Nauk SSSR (ser. fiz.) 9, 122-128 (1945). English translation: J. Phys. (USSR) 9, 249-254 (1945).
- A. J. Stam, Information and Control, vol. 2, 1959, p. 101.
- G. Folland, A. Sitaram, "The Uncertainty Principle: A Mathematical Survey", Journal of Fourier Analysis and Applications, 1997 pp 207-238.
External links
- Stanford Encyclopedia of Philosophy entry
- aip.org: Quantum mechanics 1925-1927 - The uncertainty principle
- Eric Weisstein's World of Physics - Uncertainty principle
- D. A. Arbatsky, The certainty principle
- Schrödinger equation from an exact uncertainty principle
- John Baez on the time-energy uncertainty relation
- Beating the uncertainty principle in finite-parameter systems
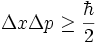
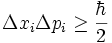
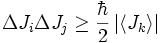
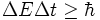
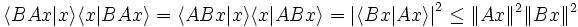
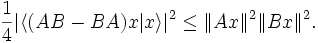
![\Delta_{\psi} A \, \Delta_{\psi} B \ge \frac{1}{2} \left|\left\langle\left[{A},{B}\right]\right\rangle_\psi\right|](Quantum uncertainty principle - Wikipedia_files/1d3f2c1271a50c638f3e2bccd2e72ff4.png)